Chemical Constituent Activities
When given a choice of vaporizing whole plant parts or oils of particular chemical constituents, but keep in mind that many plant chemical constituents are known to work together synergistically to acheive a higher degree of activity.
(+)-(E)-Caffeoyl-L-malic-acid:
antioxidant, sedative (neuro).
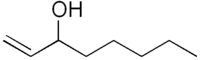 1-Octen-3-ol:
1-Octen-3-ol:
olfactic (mushroomy).
13-Hydroxyballonigrinolide:
7-Acetoxymarrubiin:
7-α-Acetoxymarrubiin (alpha-acetoxymarrubiin):
7-Glucoside:
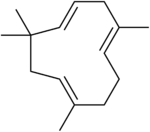 alpha-Humulene (α-humulene, humulene, α-caryophyllene [obsolete]), an isomer of β-caryophyllene:
alpha-Humulene (α-humulene, humulene, α-caryophyllene [obsolete]), an isomer of β-caryophyllene:
antiinflammatory, antitumor, gustactic, olfactic (aromatic).
 alpha-Pinene (α-pinene), boiling point = 156°C (312.8°F), one of two isomers of pinene (the other being β-Pinene):
alpha-Pinene (α-pinene), boiling point = 156°C (312.8°F), one of two isomers of pinene (the other being β-Pinene):
AChE inhibitor, allelochemic, allergenic, antiacne, antibacterial, antibiotic, antifeedant, antiflu, antiinflammatory (dosage: 500 mg/kg), antipneumonic, antineoplastic, antiseptic, antispasmodic, antistaphylococcic, antiviral, bronchodilator, cancer-preventive, coleoptophile, expectorant, herbicide (dosage: IC50=30 uM), insecticide (dosage: 0.82 uM/fly), insectifuge (dosage: 50 ppm), insectiphile, irritant, P450-2B1-inhibitor (dosage: IC50=0.087 uM), olfactic (aromatic), pesticide, sedative, spasmogenic, stimulant, tranquilizer, transdermal.
alpha-Terpineol (α-terpineol), boiling point = 217°C to 218°C (422.6°F to 424.4°F):
AChE inhibitor, antibiotic, antimalarial, antioxidant, sedative.
Alyssonoside:
Angoroside-A:
Apigenin:
11B-HSD-inhibitor, anti-ADD, anti-HIV (dosage: IC50=143 ug/ml; dosage: IC72=200 ug/ml), antiaflatoxin (dosage: IC50=2.57 ppm; Dosage: IC50=9.52 uM), antiaggregant, antiaging, antiallergic, antiangiogenic (dosage: 4 uM), antiarrhythmic, antibacterial, anticancer (Lung), anticomplementary, antidermatitic, antiestrogenic, antiherpetic (dosage: 20-54 ug/ml), antihistaminic (dosage: IC50=10-35 uM), antiinflammatory (dosage: = indomethacin; IC~65=1,000 uM), antileukemic (dosage: 20-50 uM), antimelanomic (dosage: 1-50 uM), antimetastatic (dosage: 1-50 uM), antimutagenic (dosage: ID50=0.55 ug/ml; dosage: ID50=10-40 nM), antioxidant (dosage: 1.5 x Vit. E; IC28.5=62.5 ug/ml), antiperistaltic, antiproliferant (dosage: 1-50 uM; IC50=155 uM), antispasmodic (dosage: EC50=1-5 uM), antistress (dosage: 10 mg/kg), antithyroid, antitumor (dosage: 1-50 uM), antitumor (breast) (dosage: 1-50 uM), antitumor (lung, skin), antiviral (dosage: 20-54 ug/ml), anxiolytic (dosage: 10 mg/kg), apoptotic (dosage: 12-60 uM), aromatase-inhibitor (dosage: IC65=1 uM/l), beta-Glucuronidase-inhibitor (dosage: IC50=~40 uM), CNS-depressant, COX-1-inhibitor (dosage: IC65=1,000 uM), COX-2-inhibitor (dosage: <40 uM; IC50=<15 uM; IC>65=1,000 uM), calcium-antagonist ?, cancer-preventive, choleretic, cyclooxygenase-inhibitor, cytochrome-P450-1A1-inhibitor, cytotoxic (dosage: 1-50 uM; IC50=9.5 uM; IC88=10 ug/ml), DNA-protective, deiodinase-inhibitor, differentiator (dosage: IC40=40 uM; MIC=30 uM), diuretic, estrogenic (dosage: 16% genistein; EC50=0.1-25 uM/l; EC50=1 uM), HIF-1alpha-inhibitor, hyaluronidase-inhibitor (dosage: IC50+=50-250 uM), hypotensive, ICAM-1-inhibitor, IKK-inhibitor, inotropic, interleukin-6-inhibitor, MAO-inhibitor, MAPK-inhibitor, musculotropic, mutagenic, myorelaxant, NADH-oxidase-inhibitor, NF-kB-inhibitor, NO-synthase-inhibitor (dosage: 5-50 uM), nodulation-signal, ornithine-decarboxylase-inhibitor, P21-inducer (dosage: 10-70 uM), PKC-inhibitor (dosage: IC50=10 uM), PTK-inhibitor (dosage: 10-100 uM), pesticide, polyamine-synthesis-inhibitor, progestational, protein-kinase-C-inhibitor (dosage: IC50=10-40 uM), quinone-reductase-inducer (dosage: 20 uM), radioprotective, sedative (dosage: 30-100 mg/kg), sunscreen, TNF-alpha-inhibitor (dosage: IC50=3.27 uM), topoisomerase-I-inhibitor, topoisomerase-II-inhibitor (dosage: 50 ug/ml; IC28=18 uM; IC45=180 uM), uterotrophic (dosage: EC50=0.1-25 uM/l), VEGF-inhibitor (dosage: IC50=2-7 uM), vasodilator, xanthine-oxidase-inhibitor (dosage: 0.36 uM), iNOS-Inhibitor (dosage: IC50=<15 uM).
Arenarioside:
aldose-reductase-inhibitor (dosage: IC50=2.38 uM), antibacterial, antioxidant (dosage: ED50=1.8 uM).
Ballonigrin:
Ballonigrine:
Ballotenol:
Ballotetroside:
antioxidant (dosage: ED50=7.5 uM), sedative.
Ballotinone (7-oxomarrubiin):
beta-Myrcene (β-myrcene), boiling point = 166°C to 168°C (330.8°F to 334.4°F), a noncyclic monoterpene, the most abundant terpenoid produced by cannabis:
analgesic, antiinflammatory (blocks the inflammatory activity of prostaglandin E2, via a peripheral mechanism shared by CBD, CBG, and CBC), antibiotic, antimutagenic.
 beta-Pinene (β-pinene):
beta-Pinene (β-pinene):
allergenic, antiinflammatory, antiseptic, antispasmodic, candidicide, herbicide, insectifuge, irritant, perfumery, pesticide, spasmogenic, transdermal.
borneol, boiling point = 210°C (410°F):
antibiotic.
Caffeic-acid:
aldose-reductase-inhibitor (dosage: 4 ug/ml [weak activity]), allergenic, analgesic, anti-HIV (dosage: EC50=200 ug/ml), anti-Legionella, antiadenoviral, antiaggregant, antiaging, antiatherogenic, antibacterial, anticancer, anticarcinogenic, antidepressant, antiedemic, antielastase (dosage: IC50=86 ug/ml (475 uM); IC50=93 um/l), antiescherichic, antiflu, antigonadotropic, antihemolytic (dosage: 25 uM), antihepatoadenomic (dosage: 200 ppm diet orl mus), antihepatotoxic, antiherpetic (dosage: 50 ug/ml; EC50=>50 ug/ml), antihistaminic, antihypercholesterolemic, antihyperthyroid, antiinflammatory, antileukemic, antileukotriene, antimelanogenic, antimutagenic, antinitrosaminic, antiophidic, antioxidant (dosage: 1.3 x Vit. E; 1/2 BHA; 1/3 quercetin; 30 mM; 50 uM; IC57=30 ppm), antiperoxidant (dosage: IC35=200 ug/ml; IC50=44 uM; IC85=100 ug/ml), antiproliferant, antiprostaglandin, antiradicular (dosage: 1/3 quercetin; 10 uM; 30 mM; IC50=32-35 uM), antiseptic, antispasmodic (dosage: EC50=3.4-15 uM), antistaphylococcic, antistomatitic, antisunburn, antithiamin, antithyroid, antitumor (dosage: 200 ppm diet orl mus), antitumor (skin), antitumor-promoter (dosage: IC42=10 uM), antiulcerogenic, antivaccinia, antiviral (dosage: IC50=62.5 ug/ml), anxiolytic, CNS-active, COX-2-inhibitor (dosage: IC32=100 uM), calcium-antagonist (dosage: IC50=1.2 uM rbt), cancer-preventive, carcinogenic ? (dosage: 2% [diet]), chemopreventive, cholagogue, choleretic, clastogenic, co-carcinogenic, collagen-sparing, cytoprotective, cytotoxic (dosage: TC50=200 ug/ml), DNA-active, DNA-protective, diuretic, fungicide (dosage: MIC=0.4 mg/ml), hepatocarcinogenic (dosage: 400 ppm diet orl mus [in the absence of alcohol]), hepatoprotective, hepatotropic, histamine-inhibitor, immunostimulant, insectifuge, leukotriene-inhibitor, lipoxygenase-inhibitor (dosage: IC27=5 mM; IC50=62-148 uM), lyase-inhibitor (dosage: IC50=94-164 uM), metal-chelator, ornithine-decarboxylase-inhibitor, pesticide, pro-oxidant, prostaglandigenic, sedative (dosage: 500 mg), sunscreen (dosage: IC50=2.5 mg/l; IC91=5 mg/l; IC98=25 mg/l), tumorigenic, vulnerary, xanthine-oxidase-inhibitor (dosage: IC50=39.21 uM).
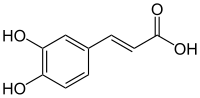
Caffeoyl-malic-acid:
Carene (delta-3-carene, Δ-3-carene), boiling point = 168°C (334.4°F), bicyclic monoterpene with a sweet pungent odor:
antiinflammatory.
 Caryophyllene (beta-caryophyllene, (-)-β-caryophyllene):
Caryophyllene (beta-caryophyllene, (-)-β-caryophyllene):
aldose-reductase-inhibitor, allergenic, analgesic, antiacne, antiasthmatic, antibacterial, anticariogenic (dosage: MIC=>1,600 ug/ml), antidermatitic, anti-edemic, antifeedant (dosage: 500 ppm), antiinflammatory (dosage: IC50=100 uM), antileishmanic, antimalarial, antionychyotic, antiproliferant, antispasmodic, antistaphylococcic, antistreptococcic, antitumor, antiulcer, candidicide, cytoprotective (gastric mucosa), FLavor (dosage: FEMA 20-200), fungicide, gastroprotective, insectifuge, irritant, larvicide, mosquitocide, olfactic (aromatic), pesticide, sedative, termitifuge.
Carvacrol:
antiinflammatory (more potent than CBG or THC; blocks the inflammatory activity of prostaglandin E2, via a peripheral mechanism shared by CBD, CBG, and CBC).
CBC (cannabichromene), boiling point = 220°C (428°F), forms when CBCA is heated or exposed to UV light:
analgesic, antibiotic, antidepressant, antifungal, antiinflammatory, antimicrobial, antiproliferative (anticancer), bone-stimulant.
CBCA (cannabichromene acid):
antibacterial, antifungal, antiinflammatory.
CBD (cannabidiol), boiling point = 160°C to 180°C (320°F to 356°F):
alerting, analgesic, antibacterial (MRSA), antibiotic, anticancer (breast), anticonvulsant, antidiabetic, antiemetic, antiepileptic, antiinflammatory, antiischemic, antiprokinetic (intestinal), antipsoriatic, antioxidant, antiproliferative (anticancer), antipsychotic, antispasmodic, anxiolytic, bone-stimulant, decreases sebum/sebocytes, immunosuppressive, neuroprotective, relieves neuropathic pain in patients with multiple sclerosis (in combination with D9-THC as a 1:1 mixture, i.e. Sativex), sedative (at exceptionally high doses), treatment of addiction, vasorelaxant.
At low doses, and a 1:1 ratio, CBD will smooth or mellow the "high" of THC, but will not eliminate it. At high doses, the "high" of THC is not interferred with by CBD. If taken first, CBD can block the "high" of THC. Since CBGA becomes CBDA and THCA by the enzymes CBDA synthase and THCA synthase, a strain with more CBDA synthase or less THCA synthase can result in CBD-rich progeny.
Cannabis strains with significant amounts of CBD:
Cannatonic (4 distinct phenotypes: phenotype 3 is purple and contains slightly more than 13% CBD at a 2:1 CBD:THC ratio; the other 3 phenotypes are [1] a very thin leaved super-sativa with >20% THC and almost zero CBD, [2] a light green, [3] a dark green)
Good Medicine (9.03% CBD and 8.2%THC, Blueberry Trainwreck [aka "Derailed Blues"] x Ogre [a special Sensi Star phenotype])
Harlequin (25 % Indica and 75% Sativa, leaves with plant in veg state at ~ 5 weeks tested 4.4% CBD and 2.13% THC)
Jamaican Lion (fem Mountain Lion [Rock Bud x Lion Heart] x m Jamaican Yarders [sativa]; ~ 8-9.7% CBD and 5.5-6.5% THC; strong sativa, the highest CBD content is found during week 8 of the its growth cycle, by week 9 the CBD/THC ratio is closer to 1:1)
Juanita la Lagrimosa (Juanita the Tearful, 8.8% CBD, 6.8% THC)
Maz's Cheese (THC: 6.41% CBD: 7.17% CBN: 0.49%)
Misty (~ 7% CBD, 10% THC)Omrita Rx3 (Rx, 2x Indica cross of Romulan Joe x Fucking Incredible, 9-12% CBD and 5-7% THC)
Sour Tsunami (Albion Sour Diesel x Tsunami; 40% Indica 60% Sativa; maturity: 9 weeks; ~ 10-11% CBD and 6-7% THC)
Atomic Jam, Black Domina, Black Queen, Bubblegum Kush, Cotton Candy x Diesel, Downtown Diesel, F5 Manawell, Granny Durkel, Intensive Care OG Kush, Jamaican Skunk, Kush, Kushage, Monkey Balls, OG Afghani, Phenom Phen, Poison OG,
Purple Diesel, Sugaree x Blue Diesel, SFV, SFVR-4?, Silver Dragon, Soma A+,
Stinky Purple, Sweet SF x OG, TB x OGK, R-4, Wu#1.
CBDA (cannabidiol acid), most cannabis plants contain less than 2% CBDA, although some strains yield plants with more CBDA than THCA, which, after heating, equals more CBD than THC. Many people prefer these strains for their many beneficial medicinal effects without the "high" associated with higher concentrations of THC. The few plants that do produce large amounts of CBDA often contain over 10% CBDA and only ~5% THCA:
antiproliferative (anticancer, antitumor).
CBDV:
anticonvulsant, bone-stimulant.
CBDVA:
CBG (cannabigerol):
analgesic, antibacterial (MRSA), antibiotic, anticancer (prostate), antidepressant, antifungal, antiinflammatory, antipsoriasis, antiproliferative (anti-tumor, human epithelial cancer tumors), bone-stimulant.
CBGA (cannabigerol acid), the primary cannabinoid from which all others derive, CBGA becomes CBDA and THCA by the enzymes CBDA synthase and THCA synthase:
analgesic, antiinflammatory.
CBL (cannabicyclol), forms when CBLA is heated or exposed to UV light:
CBLA (cannabicyclol acid), forms when CBCA absorbs UV light:
CBN (cannabinol), boiling point = 185°C (365°F), oxidation breakdown product, from THC:
antibacterial (MRSA), antibiotic, anticancer (breast), burns, psoriasis, psychoactive (mildly), sedative.
CBNA (cannabinol acid), a breakdown product of THCA by air oxidation:
 Chlorogenic-acid:
Chlorogenic-acid:
aldose-reductase-inhibitor (dosage: IC50=1.8 uM rat [strong activity]), allelochemic, allergenic, analgesic, anti-EBV, anti-HIV, anti-Legionella, antiatherosclerotic, antibacterial, anticancer [colon, fore-stomach, liver, skin]), anticarcinogenic, antidiabetic, antifeedant, antigenotoxic, antigonadotropic, antihemolytic (dosage: 10 uM), antihepatotoxic, antiherpetic, antihistaminic, antihypercholesterolemic, antihyperthyroid, antiinflammatory, antileukotriene, antimelanogenic, antimutagenic, antinitrosaminic, antioxidant (dosage: IC50=54.2 uM; IC53=200 ppm; IC80=12 uM), antiperoxidant (dosage: IC50=36 uM), antipolio, antiradicular (dosage: 10 uM; 9 x quercetin), antiseptic, antisunburn, antithyroid, antitumor, antitumor (colon, fore-stomach, liver, skin), antitumor-promoter (dosage: IC25=10 uM), antiulcer, antiviral, autotoxic, CNS-active, CNS-stimulant (dosage: 1/6 Caffeine), cancer-preventive, cardioprotective, chemopreventive, cholagogue, choleretic, clastogenic, collagen-sparing, diuretic, fungicide, hepatoprotective, histamine-inhibitor, hypoglycemic, immunostimulant, insectifuge, interferonogenic, juvabional, larvistat, leukotriene-inhibitor, lipoxygenase-inhibitor (dosage: IC23=5 mM), metal-chelator, NO-genic, ornithine-decarboxylase-inhibitor, oviposition-stimulant, pesticide, sunscreen, sweetener, vulnerary.
 Choline:
Choline:
antialzheimeran (dosage: 5-16 g/man/day), antichoreic, anticirrhotic (dosage: 6,000 mg/man/day), anticystinuric, antidementia, antidiabetic, antidyskinetic (dosage: 150-200 mg/kg/man/day), antihomocysteine, antimanic (dosage: 15-30 g/man/day/orl), antinociceptive, antisteatotic, cardiodepressant, cerebrotonic, cholinergic, hepatoprotective, hypotensive, ileorelaxant, lipotropic, memorigenic, parasympathomimetic (dosage: (1/1,000th acetylcholine)).
 α-Copaene (alpha-copaene, copaene):
α-Copaene (alpha-copaene, copaene):
carminative.
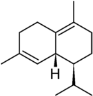 δ-Cadinene (delta-cadinene):
δ-Cadinene (delta-cadinene):
aldose-reductase-inhibitor, antiacne, antibacterial (dosage: MIC800 ug/ml), anticariogenic (dosage: MIC800 ug/ml), antistreptococcic, cytochrome-P450-inducer, P450-inducer, pesticide, testosterone-inducer.
delta-Limonene (δ-limonene), boiling point = 177°C (350.6°F):
antidepressant, antimutagenic, cannabinoid agonist?, immune potentiator.
D8-THC (Δ8–THC = Δ8-tetrahydrocannabinol), boiling point = 175°C to 178°C (347°F to 352°F):
effects thought to be very similar to Δ9-THC but less psychoactive and more stable, antiemetic.
ESSENTIAL OIL:
Eucalyptol (1,8-cineole), boiling point = 176°C (348.8°F):
AChE inhibitor, antibiotic, antiinflammatory, antinociceptive, antiviral, increases cerebral blood flow, stimulant.
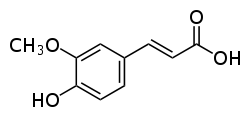 Ferulic-acid:
Ferulic-acid:
allelopathic, analgesic, antiaggregant, antiallergic, antiarrhythmic, antibacterial, anticancer (colon, forestomach, liver, skin), anticarcinogenic, antidysmenorrheic, antiestrogenic, antihepatotoxic, antiherpetic, antiinflammatory, antileukemic (dosage: IC50=25-56 ug/ml), antimitotic, antimutagenic, antineoplastic (dosage: [stomach]), antinitrosaminic, antioxidant (dosage: 1/2 BHA; 1/3 quercetin; 3,000 uM; EC50=9-15 ug/ml; IC51=200 ppm), antiradicular (dosage: EC50=9-15 ug/ml; IC50=116-124 uM), antiserotonin, antispasmodic, antithrombic, antitumor, antitumor (colon, forestomach, liver, skin), antitumor-promoter (dosage: IC46=10 uM), antiviral, arteriodilator, cancer-preventive, candidicide, cardiac, cholagogue, choleretic, fungicide, hepatoprotective, hepatotropic, herbicide, hydrocholerectic, hypolipidemic, immunostimulant, insectifuge, metal-chelator, ornithine-decarboxylase-inhibitor, pesticide, phagocytotic, preservative, prostaglandigenic, prostaglandin-synthesis-inhibitor (dosage: 0.58-3.2 mM), sunscreen, uterosedative (dosage: 30-100 mg/kg ivn rat).
 Forsythoside-B:
Forsythoside-B:
aldose-reductase-inhibitor (dosage: IC50=4.83 uM), antiinflammatory, antioxidant (dosage: ED50=1 uM), antisepsis, neuroprotective (dosage: >8 mg/kg produced a significant neuroprotective potential in cerebral ischemia and reperfusion rats).
Gallic-acid:
ACE-inhibitor (dosage: IC50=7.7 mM/l), analgesic, anti-HIV, anti-MRSA, antiadenovirus, antiallergenic, antianaphylactic, antiangiogenic, antiasthmatic, antibacterial (dosage: MIC=1,000 ug/ml), antibronchitic, anticancer, anticarcinomic (dosage: ED50=3), antiescherichic, antifibrinolytic, antiflu, antihepatotoxic, antiherpetic (dosage: EC50=>10 ug/ml), antiinflammatory, antileishmanic (dosage: EC50=4.4 ug/ml), antimutagenic, antinitrosaminic, antioxidant (dosage: 2/3 BHA; 7 x quercetin; IC44=33 ppm), antiperiodontitic (dosage: 10 ug/ml), antiperoxidant (dosage: IC50=69 uM), antipolio, antiproteolytic (dosage: 10 ug/ml), antiradicular (dosage: 7 x quercetin; IC50=4.9 uM), antiseptic, antistaphylococcic (dosage: MIC=1,000 ug/ml), antitumor, antitumor-promoter, antiviral, apoptotic, astringent, bacteristat, bronchodilator, cancer-preventive, candidicide, carcinogenic, choleretic, cyclooxygenase-inhibitor, cytotoxic (dosage: 500 uM), floral-inhibitor, gram(+)icide (dosage: MIC=1,000 ug/ml), gram(-)icide (dosage: MIC=1,000 ug/ml), hemostat, hepatoprotective, immunomodulator, immunostimulant, immunosuppressant, insulin-sparing, myorelaxant, NO-inhibitor (dosage: IC-26=250 uM), nephrotoxic, pesticide, styptic, topoisomerase-I-inhibitor, xanthine-oxidase-inhibitor (dosage: IC50=24 uM).
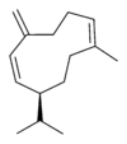 Germacrene-D:
Germacrene-D:
pesticide, pheromone.
Lavandulifolioside:
Linalool, boiling point = 198°C (388.4°F):
acaricide (dosage: C90=<15 ul/l [=<15 ppm]), allergenic, analgesic, anesthetic (dosage: 0.01-1 ug/ml), antiacetylcholinesterase, antiallergic, antianaphylactic, antibacterial (dosage: MIC=1,600 ug/ml), anticariogenic (dosage: MIC=1,600 ug/ml), anticolic, anticonvulsant (dosage: 200 mg/kg ipr mus), antiedemic, antidepressant, antiglutamaergic, antihistaminic, antiinflammatory, antileukemic (dosage: IC50=3.5-4.2 ug/ml), antilymphomic (dosage: IC50=3.5-4.2 ug/ml), antimange, antimite, antimutagenic, antiseptic (dosage: 5 x phenol), antishock, antispasmodic, antiviral, anxiolytic, barbituate-synergist, bronchorelaxant, CNS-depressant, cancer-preventive, candidistat, carminative, culicide (dosage: LC50=156-194 mg/l), cytotoxic, expectorant, FLavor (dosage: FEMA 2-40), fungicide, GABA-nergic, hypnotic, hypothermic, immune potentiator, insecticide (dosage: LC50=156-194 mg/l), insectifuge, irritant, larvicide (dosage: LC50=156-194 mg/l), mosquitofuge (dosage: > Deet), motor-depressant, nematicide (dosage: MLC=1 mg/ml), perfumery, pesticide, prooxidant, sedative (dosage: 200 mg/kg ipr mus (1% as active as diazepam); ED=1-32 mg/kg), termitifuge, trichomonicide (dosage: LD100=600 ug/ml), tumor-promoter.
Marrubiin (LD50=370 mg/kg):
antiarrhythmic, antinociceptive, arrhythmigenic, bitter, cardioactive, choleretic, expectorant, secretogogue.
p-Cymene, boiling point = 177°C (350.6°F):
AChE inhibitor, antibiotic, anticandidal.
Preleosibirin:
Pulegone, boiling point = 224°C (435.2°F):
AChE inhibitor, antipyretic, memory booster?, sedative.
Raffinose:
flatugenic.
Sabinene:
antibacterial, antihelicobacter, antiseptic, antiulcer, perfumery.
Stachyose:
flatugenic.
Sucrose:
aggregant, antihiccup (dosage: 1 tsp), antiophthalmic, antioxidant, atherogenic, collyrium, demulcent, flatugenic, hypercholesterolemic, preservative, sweetener (dosage: 1 x sucrose), triglycerigenic, uricogenic, vulnerary.
Tageretin:
antineoplastic, fungicide, pesticide.
Tannin:
anthelmintic, anti-HIV, antibacterial, anticancer, anticariogenic, antidiarrheic, antidysenteric, antihepatotoxic, antihypertensive, antilipolytic, antimutagenic, antinephritic, antiophidic, antioxidant (dosage: 1/3 quercetin; IC50=1.44 ug/ml), antiradicular (dosage: 1/3 quercetin; 500 mg/kg/day orl mus), antirenitic, antitumor, antitumor-promoter, antiulcer, antiviral, cancer-preventive, carcinogenic, chelator, cyclooxygenase-inhibitor, glucosyl-transferase-inhibitor, hepatoprotective, immunosuppressant, lipoxygenase-inhibitor, MAO-inhibitor, ornithine-decarboxylase-inhibitor, pesticide, psychotropic ?, xanthine-oxidase-inhibitor.
Terpineol-4-ol, boiling point = 209°C (408.2°F):
AChE inhibitor, antibiotic.
THC (Δ9-THC = D-9-THC = Delta-9-tetrahydrocannabinol)... boiling point = 157°C (315°F); the most abundant cannabinoid (usually, otherwise cannabidiol, depends on particular plant); the more abundant of the two psychoactive
of the cannabinoids (the other is Delta-8-THC):
analgesic, anti-Alzheimers, antiemetic, antiinflammatory, antioxidant, antipruritic, antispasmodic, appetite stimulant, beneficial for duodenal ulcers, bronchodilatory, euphoriant, muscle relaxant, pain reliever.
THCA (Δ9-THCA = Δ9-THCA A = Δ9-tetrahydrocannabinol acid) the precursor to THC; a large fraction (at most about 70%) of the THCA converts to THC upon strong heating (> 200°F) or exposure to UV light:
analgesic, antiinflammatory, antiproliferative (anticancer), antispasmodic, appetite stimulant, neuroprotector.
THCA-C4 (tetrahydrocannabinol-C4):
effects thought to be similar to THCA.
THV (THCV, tetrahydrocannabivarin), boiling point = <220°C (<428°F), formed when THVA is heated or exposed to UV
light; increases the onset and intensity of THC effects, but also causes the high to end sooner; found primarily in strains of African and Asian cannabis:
analgesic, anorectic, anticonvulsant, antiepileptic, beneficial in treating metabolic syndrome, bone-stimulant, euphoriant.
THVA (THCVA = tetrahydrocannabivarin acid):
effects thought to be similar to THCA.
Verbascose:
flatulent.
Verbascoside:
aldose-reductase-inhibitor (dosage: IC50=0.39-15.1 uM), analgesic, antibacterial, antifeedant, antihepatotoxic, antihypertensive, antiinflammatory, antileukemic (dosage: ED50=2.6 ug/ml), antioxidant (dosage: ED50=1 uM), antiseptic, antistaph, antitumor, cytotoxic (dosage: ED50=2.6 ug/ml), fungicide (dosage: MIC=0.4 mg/ml), hypertensive, immunosuppressant, lipoxygenase-Inhibitor, PKC-inhibitor, pesticide, phytoalexin.
Vicenin-2:
ACE-inhibitor (dosage: IC50=0.2 mM/l), antiangiogenic, anticancer, anticancer (prostate), anticarcinomic, antiinflammatory (dosage: ED50=148 (ipr mus)), antiproliferant, apoptotic, cyclin-B1-inhibitor, cyclin-D1-inhibitor, oviposition-stimulant, radioprotective.
References:
Acta Botanica Sinica, 32; 49 p.
Andary, C.; Caffeic Acid Glycoside Esters and Pharmacology; Polyphenolic Phenom, 1993; 237-245 pp.
ANON; 1948-1976; The Wealth of India raw materials; Publications and Information Directorate, CSIR, New Delhi; 11 volumes.
Antiviral Research, 14; 323 p.
Atta-ur-Rahman; ed.; Studies in Natural Products Chemistry; Volume 33, Bioactive Natural Products (Part M), 2006; 679, 680 pp.
Azuma, Y.; Onishi, Y.; Sato, Y.; Kizaki, H.; Effects of Protein Tyrosine Kinase Inhibitors with Different Modes of Action on Topoisomerase Activity and Death of IL-2-Dependent CTLL-2-Cells; J Biochemistry, 118, 1995; 312-318 pp.
Benson, John A. Jr.; Joy, Janet E.; and Watson, Stanley J. Jr.; eds; Marijuana and Medicine, Assessing the Science Base; Division of Neuroscience and Behavioral Health; Institute of Medicine; 1999.
Biochimica & Biophysica Acta, 1115, 1991; 69 p.
Bisset, N.G., ed.; Herbal Drugs and Phytopharmaceuticals; CRC Press, Boca Raton, FL, 1994; 566 pp.
Blaschek, W.; Hansel, R.; Keller, K.; Reichling, J.; Rimpler, H.; and Schneider, G.; eds.; . Hager's Handbuch der Pharmazeutischen Praxis, Auflage Band 2 (A-K), 909 pp., (L-Z), 858 pp. Springer-Verlag, Berlin, 1998.
Buchbauer, G.; Jirovetz, L.; Nikiforov, A.; Remberg, G.; Raverdino, V.; Headspace-Analysis and Aroma Compounds of Austrian Hay-Blossoms (Flores Graminis, Graminis Flos) used in Aromatherapy; J. Ess. Oil Res., 2; 1989, 1990; 185-191 pp.
Buchbauer et al; Therapeutic properties of essential oils and fragrances, Chap.12 in Teranishi,R; Buttery, R.G; and Sugisawa,H.; eds.; Bioactive Volatile Compounds from Plants, ACS Symposium Series 525; Amer. Chem. Soc., Washington DC; 1993.
Cancer Research, 48; 5941 p.
Castleman, Michael; The Healing Herbs; Rodale Press, Emmaus, PA, 1991; 436 pp.
Chang, H. M.; Yeung, H. W.; Tso, W.; and Koo, A.; eds.; Advance in Chinese Medicinal Materials Research; World Scientific Publishing Co., Philadelphia Pa., 1985; 210 p.
Charalambous, G.; ed.; Spices, Herbs and Edible Fungi; Elsevier Science B. V. Amsterdam; 1994; 764 p.
Chiang, L. C.; Chiang, W.; Chang, M. Y.; Ng, L. T.; Lin, C. C.; Antileukemic activity of selected natural products in Taiwan; Am J Chin Med, 31(1), 2003; 37-46 pp.
Daels-Rakotoarison, D. A.; Seidel, V.; Gressier, B.; Brunet, C.; Tillequin, F.; Bailleul, F.; Luyckx, M.; Dine, T.; Cazin, M.; and Cazin, J. C.; Neurosedative and antioxidant activities of phenylpropanoids from ballota nigra. Arzneimittelforschung 2000; 50(1):16-23.
Davies, S.; and Stewart, A.; Nutritional Medicine; Avon Books, New York, 1990; 509 p.
Dorsch, W.; Wagner, H.; New Antiasthmatic Drugs from Traditional Medicine?; Int Arch Allergy Appl Immunol 94, 1991; 262-265 pp.
Dr. Duke's Phytochemical and Ethnobotanical Databases; [Online Database] 21 February 2012.
Economic & Medicinal Plant Research; 1: 53, 124 pp; 5: 194, 195, 197, 207, 363 pp; 6: 189, 235 pp.
Fernandes E.S.; Passos G.F.; Medeiros R.; da Cunha F.M.; Ferreira J.; Campos M.M.; Pianowski L.F.; Calixto J.B.; Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea; European Journal of Pharmacology 569 (3), 2007; 228–236 pp.
Frank, Mel; Marijuana Growers Guide, Fourth Edition; Red Eye Press; 1996.
Friedman, M.; and Dao, L.; Effect of Autoclaving and Conventional and Microwave Baking on the Ergot Alkaloid and Chlorogenic Acid Contents of Morning Glory (Ipomoea tricolor Cav. cv.) Heavenly Blue Seeds; J. Agric. Food Chem., 38, 1990; 805-808 pp.
Hagerman, A.E.; Tannin-Protein Interactions, Phenolic Compounds in Food and their Effects on Health, Ch. 19.
Halent Laboratories, Cannabinoids Primer.
Hamada, S. I.; Kataoka, T.; Woo, J. T.; Yamada, A.; Yoshida, T.; Nishimura, T.; Otake, N.; Nagai, K.; Immunosuppressive Effects by Gallic Acid and Chebulagic Acid on CTL-Mediated Cytotoxicity; Biol Pharm Bull, 20, 1997; 1017-1019 pp.
Harborne, Jeffery B.; and Baxter H.; eds.; Phytochemical Dictionary, A Handbook of Bioactive Compounds from Plants; Taylor & Frost, London, 1983; 791 p.
Hatano, T.; Yasuhara, T.; Yoshihara, R.; Agata, I.; Noro, T.; and Okuda, T.; Effects of Interaction of Tannins with Co-existing Substances, VII, Inhibitory Effects of Tannins Related Polyphenols on Xanthine Oxidase; Chem. Pharm. Bull.; 38(5), 1989; 1224-1229 pp.
HerbalGram No. 22, Spring 1990; 14 p.
Holappa, L.D.; and Blum, U.; Effects of Exogenously Applied Ferulic Acid, a Potential Allelopathic Compound, on Leaf Growth, Water Utilization, and Endogenous Abscisic Acid Levels of Tomato, Cucumber, and Bean; J. of Chemical Ecology, 17(5), 1991; 865 p.
http://projectcbd.com
Huang, K. C.; The Pharmacology of Chinese Herbs; CRC Press, Boca Raton, FL, 1993; 388 p.
Huang, M.T.; Ho, C.T.; and Lee, C.Y.; eds; Phenolic Compounds in Food and their Effects on Health; Antioxidants & Cancer Prevention, ACS Symposium Series 507, ACS, Washington, 1992; 402 pp.
Huang, M.T.; and Ferraro, T.; Phenolic Compounds in Food and Cancer Prevention, Phenolic Compounds in Food and Their Effects on Health, Ch. 2, 21 p.
Ichikawa, K., et al.; Isolation and Structure Determination of Aldose Reductase Inhibitors from Traditional Thai Medicine, and Syntheses of their Derivatives; Sankyo Kenkyusho Nempo, 43, 1991; 99-110 pp.
Izzo, Angelo A.; Borrelli, Francesca; Capasso, Raffaele; Di Marzo, Vincenzo; and Mechoulam, Raphael; Non-Psychotropic plant cannabinoids: new therapuetic opportunities from an ancient herb, Trends in Pharmacological Sciences, Vol. 30 (2009) pp. 515-527.
Jacobson, M.; Glossary of Plant-Derived Insect Deterrents; CRC Press, Inc., Boca Raton, FL, 1990; 213 p.
Jiang, WL; Tian, JW; Fu, FH; Zhu, HB; Hou J.; Neuroprotective efficacy and therapeutic window of Forsythoside B: in a rat model of cerebral ischemia and reperfusion injury; Eur J Pharmacol.; 2010; 640(1-3); 75-81 pp.
Jiang, W.-L.; Yong-Xu; Zhang, S.-P.; Zhu, H.-B.; and Jian-Hou; (2011); Forsythoside B Protects Against Experimental Sepsis by Modulating Inflammatory Factors; Phytotherapy Research; doi: 10.1002/ptr.3668.
Jiang, W-L; Fu, F-H; Xu, B-M; Tian, J-W; Zhu,H-B; Jian-Hou; Cardioprotection with forsythoside B in rat myocardial ischemia-reperfusion injury: relation to inflammation response; Phytomedicine international journal of phytotherapy and phytopharmacology (2010) Volume: 17, Issue: 8-9, 635-639 pp.
Joseph, J.; Nadeau, D.; and Underwood, A.; The Color Code; Hyperion, NY, 2001.
Journal of Medicinal Food 2, 1999; 227, 235 p.
Keeler, R.F.; and Tu, A.T.; eds.; Toxicology of Plant and Fungal Compounds, (Handbook of Natural Toxins Vol. 6); Marcel Dekker, Inc. NY, 1991; 665 pp.
Ki Soon Rhee; Oilseed Food Ingredients Used to Minimize Oxidative Flavor Deterioration in Meat Products, Phenolic Compounds in Food and their Effects on Health, Ch. 18.
Kohda, H.; Tanaka, S.; Yamaoka, Y.; Yahara, S.; Nohara, T.; Tanimoto, T.; Tanaka, A.; Studies on Lens-Aldose-Reductase Inhibitor in Medicinal Plants; II Active Constituents of Monochasma savatierii FRANCH, et MAXIM; Chem. Pharm. Bull.; 37(11); 1989; 3153-3154 pp.
Lazarova, G.; Kostova, I.; Neychev, H.; Photodynamic damage prevention by some hydroxycoumarins; Fitoterapia 64(2), 1992, 1993; 134-136 pp.
Lawrence Review of Natural Products, June 88; June 1990.
Leung, A. Y. and Foster, S; Encyclopedia of Common Natural Ingredients, 2nd Ed.; John Wiley & Sons, New York, 1995; 649 p.
Martindale's 28th
Martindale's 29th
McEvily, A.J.; Iyengar, R.; and Gross, A.T.; Inhibition of Polyphenol Oxidase by Phenolic Compounds, Phenolic Compounds in Food and their Effects on Health, Ch. 25.
McKenna, D. J.;Hughes, K.; and Jones, K.; Green Tea Monograph, Alternative Therapies, 6(3), 2000; 61-82 pp.
McPartland John M, Russo Ethan B. (2001). Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?. Journal of Cannabis Therapeutics. 1(3/4):103-132.
Merck 11th Edition
Mills, Simon; and Bone, Kerry; Phytotherapy; Churchill Livinston, Edinburgh, 2000.
Muroi, H.; and Kubo, I.; Combination Effects of Antibacterial Compounds in Green Tea Flavor against Streptococcus mutans; J. Agric. Food Chem., 41, 1993; 1102-1105 pp.
Newall, C. A.; Anderson, L. A.; and Phillipson, J. D.; Herbal Medicine - A Guide for Healthcare Professionals; The Pharmaceutical Press, London, 1996; 296 p.
Nigg, H.N.; and Seigler, D.S.; eds.; Phytochemical Resources for Medicine and Agriculture; Plenum Press, New York, 1992; 445 p.
Neuwinger, H. D.; African Ethnobotany - Poisons and Drugs; Chapman & Hall, New York, 1996; 941 p.
Ohnishi, M.; Morishita, H.; Iwahashi, H.; Toda, S.; Shirataki, Y.; Kimura, M.; and Kido, R.; Inhibitory Effects of Chlorogenic Acids on Linoleic Acid Peroxidation and Haemolysis; Phytochemistry, 36(3), 1993, 1994; 579-583 pp.
Okuda, T.; Yoshida, T.; and Hatano, T.; Antioxidant Effects of Tannins and Related Polyphenols, Phenolic Compounds in Food and their Effects on Health, Ch.7, 93 p.
Oszmianski, J.; and Lee, C.Y.; Inhibitory Effect of Phenolics on Carotene Bleaching in Vegetables; J. Agric. Food Chem., 38, 1990; 688-690 pp.
Phenolic Compounds in Food and their Effects on Health, 69 p.
Phytotherapy Research, 4; 73 p.
Pizzorno, J.E.; and Murray, M.T.; A Textbook of Natural Medicine; John Bastyr College Publications, Seattle, Washington, 1985.
Planta Medica, 56, 1990; 638 p.
Planta Medica, 57, 1991; A54, A113.
Recio, M. C.; Rios, J. L.; and Villar, A.; A review of some antimicrobial compounds isolated from medicinal plants reported in the literature 1978-1988, Phytotherapy Research, 3(4), 1989, 117-125 pp.
Revista Itiliana Eppos, 12, 1994; 5 p.
Santti, R.; Makela, S.; Strauss, L.; Korman, J.; Kostian, M. L.; Phytoestrogens: Potential Endocrine Disruptors in Males; Toxicol Ind Health, 14, 1998; 223-237 pp.
Seidel, V.; Verholle, M.; Malard, Y.; Tillequin, F.; Fruchart, J. C.; Duriez, P.; Bailleul, F.; and Teissier, E.; Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation; Phytother Res 14(2), 2000; 93-98 pp.
Shimizu,M.; et al; Anti-inflammatory Constituents of Topically Applied Crude Drugs, IV, 1 (Constituents and Anti-inflammatory Effect of Paraguayan Crude Drug "Alhucema" (Lavandula latifolia Vill.)2). Chem. Pharm. Bull. 38(8), 1990; 2283-2284 pp.
Shoyakugaku Zasshi; 44; 183 p.
Singh, J.; Gupta, K.; and Arora, S.K.; Changes in the anti-nutritional factors of developing seeds and pod walls of fenugreek (Trigonella foenum graecum L.); Plant Foods for Human Nutrition; 46, 1993, 1994; 77-84 pp.
Stitt, P. A.; Why George Should Eat Broccoli; Dougherty Co, Milwaukee, WI, 1990, 399 pp.
Stored, J.; Prod. Res., 22, 1986; 141 p.
Taylor, Leslie; The Healing Power of Rainforest Herbs; SquareOne Publisher, Garden City Park, NY, 2005; 519 pp.
Tunon, H.; Thorsell, W.; and Bohlin, L.; Mosquito Repelling Activity of Compounds Occurring in Achillea millefolium L. (Asteraceae), Economic Botany; 48(2), 1993, 1994; 111-120 pp.
Uchida, U.; Ohta, H.; Niwa, M.; Mori, A.; Nonaka, G-i.; Nishioka, I.; and Zaki, M.; Prolongation of Life Span of Stroke-Prone Spontaneously Hypertensive Rats (SHRSP) Ingesting Persimmon Tannin; Chem. Pharm. Bull.; 38(4); 1989, 1990; 1049-1052 pp.
Uda, Y.; Price, K. R.; Williamson, G.; Rhodes, M. J. C.; Induction of the Anticarcinogenic Marker Enzyme, Quinone Reductase, in Murine Hepatoma Cells In Vitro by Flavonoids; Cancer Lett., 120 (2), 1997; 213-216pp.
Ueno, H.; Horie, S.; et al; Chemical And Pharmaceutical Studies On Medicinal Plants In Paraguay: Geraniin, An Angiotensin-Converting Enzyme Inhibitor From "Paraparai Mi," Phyllanthus niruri; J. of Natural Products, 51(2), 1988; 357-359 pp.
Vlietinck, A.J.; and Dommisse, R.A.; eds.; Advances in Medicinal Plant Research; Wiss. Verlag. Stuttgart; 1985.
Wagner & Wolff, eds.; New Natural Products; RS164. I56. 1977; 176 p.
Watt, J.M.; and Breyer-Brandwijk, M.G.; The Medicinal and Poisonous Plants of Southern and Eastern Africa; 1962.
Wichtl, M.; Ein Handbuch fur Apotheker und Arzte (Note: English translation); Wissenschaftliche Verlagsgesellscharft, Teedrogen, mbH Stuttgart, 1984, 1989; 393 p.
Williamson, E. M.; and Evans, F. J.; Potter's New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed.; Saffron Walden, the C. W. Daniel Co., Ltd., Essex UK, 1988, reprint 1989; 362 p.
Yoshikawa, M.; et al; Medicinal Flowers. I. Aldose Reductase Inhibitors and Three New Eudesmane-Type Sesquiterpenes, Kikkanols A, B, and C, From the Flowers of Chrysanthemum inducum L. Chem Pharm Bull; 47(3), 1999; 340-345 pp.
Zebovitz, T. C. ed.; Part VII, Flavor and Fragrance Substances; in Keith L. H. and Walters, D.B., eds; Compendium of Safety Data Sheets for Research and Industrial Chemicals; VCH Publishers, New York, 1989; 3560-4253 pp.
Zheng, G-Q.; Kenney, P.M.; and Lam, L.K.T.; Sesquiterpenes from Clove (Eugenia caryophyllata) as Potential Anticarcinogenic Agents; Journal of Natural Products 55(7), 1992; 999-1003 pp.
Vaporize your weed, you won't regret it!
Visit the Headshop... BUY A VAPORIZER NOW!




 Copyright © 2014
Copyright © 2014